CALCUTIONS AREA
CLICK ON CALCULATOR
Infant Data
Postmenstrual Age(PMA) = gestational age plus postnatal age.
Results
MEDICAL INFORMATIONS
INDICATIONS
Drug of choice for serious infections caused by methicillin-resistant staphylococci (eg, S aureus and S epidermidis) and penicillin-resistant pneumococci.
- Anthrax:

- Clostridium difficileinfection: Young children and infants may be asymptomatically colonized, but are unlikely to be infected with C difficile. Routine testing for C difficile in neonates or infants 12 months or younger with diarrhea is not recommended.
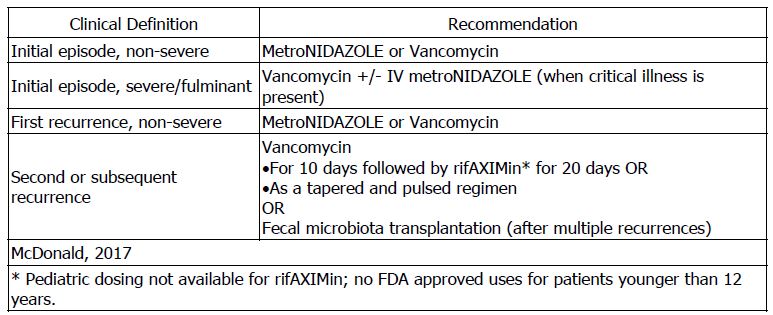
- Infective Endocarditis: The following recommendations are based on a consensus of experts. The full pediatric guidelines can be found here:

- Sepsis, Prophylaxis; Catheter Removal: Clinical sepsis rates of 2% were observed when infants were receiving antibiotics within 12 hours of removing a peripherally inserted central catheter (PICC) compared with 13% (p=0.03) of infants not on antibiotics within 12 hours of removal in retrospective chart review (n=196 premature infants). Elective vancomycin 15 mg/kg IV was administered 2 hours prior to catheter removal in 27 out of 48 removals. The duration of PICC lines was 24.3 days (range, 8 to 67 days). Susceptibility pattern for vancomycin did not change during the study period. Reductions (11% vs 0%; p=0.021) in culture-confirmed sepsis were demonstrated in a prospective randomized controlled study in 88 preterm infants administered cefazolin 1 hour prior to and 12 hours after removal of a PICC line compared with no antibiotic use. However, this study was criticized for methodology shortcomings that limit its applicability. Sepsis rates were 10.3% with removal of a PICC without antibiotics 48 hours prior to removal compared with 1.5% (p=0.002) in neonates on cefazolin/gentamicin at the time of removal of the PICC in a retrospective study (n=345)
- Sepsis: There was no difference in failure rate between a 7-day vs 10-day duration of empiric treatment with IV cefTRIAXone and amikacin for culture-proven sepsis in 132 neonates, 1.5 kg or more and gestational age 32 weeks or more, who remitted clinically by day 5 in a randomized study. The follow-up period was 28 days. The median age at presentation was 3 days (2 to 4 days) and 56.8% had early-onset sepsis. The majority of organisms in blood cultures were Klebsiella spp. (40.9%), Staphylococcus aureus (22.7%), Enterobacter spp. (16.7%), and MRSA (7.6%).
- Ventriculitis, Device Associated: All 7 preterm infants (less than 28 weeks gestation) experienced resolution of ventriculitis with intraventricular vancomycin (5 out of 8 events were treated with additional IV vancomycin) in a case series. Ventriculitis resolved in a median of 5.5 days (range, 2 to 31 days). A total of 40 intraventricular vancomycin doses (3, 5, 10, or 15 mg) were administered in 8 ventriculitis events. Intraventricular vancomycin was administered over 2 minutes as a sterile 10-mg/mL solution at the end of the normal reservoir tap followed by a 1-mL sterile NS flush of the ventriculostomy reservoir and catheter. Doses were repeated for CSF concentrations less than 10 mg/L. The longest intervals to maintain CSF vancomycin concentration above 10 mg/L were 45 hours for 3 mg (12.8 mg/L), 97 hours for 5 mg (21.4 mg/L), and 114 hours for 10 mg (19.5 mg/L). Only 2 CSF concentrations were available for the 15-mg dose; 230.7 mg/L at 24 hours post-dose and 44.9 mg/L at 68 hours post-dose. Concomitant IV vancomycin was used in 5 of the 8 events; median vancomycin trough was 6.1 mg/L (range, less than 2 to more than 100 mg/L). Adverse effects due to intraventricular vancomycin were not confirmed. One patient, with maximum vancomycin CSF concentrations of 24.9 mg/L, experienced bilateral reduced hearing which necessitated hearing aids. Daily measurement of vancomycin CSF concentrations are suggested in patients receiving intraventricular vancomycin
FDA APPROVED INDICATION
Clostridium difficile-associated diarrhea and staphylococcal enterocolitis in pediatric patients younger than 18 years of age. Not effective by the oral route for any other infection. Specific neonatal data were not provided by the manufacturer.
ADVERSE EFFECTS
- Nephrotoxicity and ototoxicity: May be enhanced by aminoglycoside therapy. The overall rate of acute kidney injury (AKI) was 2.7% in a retrospective chart review in a neonatal intensive care unit (n=110; mean gestational age 29 weeks, mean birth weight 1200 g). The incidence of AKI increased with higher vancomycin trough concentrations (p=0.04); 1.38% for less than 10 mg/L, 0% for 10 to 15 mg/L, and 18.18% for greater than 15 mg/L. The use of concurrent furosemide and vancomycin was associated with an increased risk of acute kidney injury (AKI) (adjusted OR, 3.52; 95% CI, 1.88 to 6.62) in pediatric patients (0 to 18 years of age) in the intensive care unit in a retrospective study (n=265). The rate of AKI was 23.4%
- Rash and hypotension (red man syndrome): Appears rapidly and resolves within minutes to hours. Lengthening infusion time usually eliminates risk for subsequent doses.
- Neutropenia: Reported after prolonged administration (more than 3 weeks).
- Phlebitis: May be minimized by slow infusion and dilution of the drug.
ADMINISTRATION
- Intravenous: Administer by intermittent IV infusion over 60 to 120 minutes (no more than 10 mg/minute) at a concentration not to exceed 5 mg/mL. Concentrations up to 10 mg/mL have been used in fluid restricted patients . Some institutions use standard concentrations of 5 and 10 mg/mL.
- Extravasation Management: reports/case series (n=237) and are not specific to vancomycin extravasation; subcutaneous saline irrigation with or without hyaluronidase infiltration was commonly used. No standardized management was established. An option for more severe injuries (stages 3 and 4) is subcutaneous irrigation with saline, but this is not advocated as standard treatment. Conservative management is appropriate for mild extravasation (stages 1 and 2). Although not neonatal-specific, the following are recommendations for extravasation of acidic or alkaline agents (vancomycin is acidic with a pH of 4).
- General:
- Stop and disconnect infusion; do not remove the cannula or needle.
- Attempt to gently aspirate as much extravasated agent as possible; avoid manual pressure.
- Remove cannula or needle.
- Dry heat and elevation.
- Closely monitor for signs of coagulation and ischemia.
- Avoid attempt at pH neutralization (vancomycin – pH 4).
- Monitor and consider the need for surgical management such as surgical flushing with normal saline or debridement and excision of necrotic tissue (especially if pain persists for 1 to 2 weeks). In cases of compartment syndrome, surgical decompression may be required.
- Refractory Events:
- Hyaluronidase 15 units intradermally along injection site and edematous area. Give as five, 0.2-mL intradermal injections along extravasation site and edematous tissue.
- Inadvertent Intraarterial Administration:
- Leave inadvertent intraarterial line in place for diagnostics.
- Systemic heparin titrated to therapeutic anticoagulant effect.
- Stellate ganglion block.
- General:
MONITORING
- Auditory Function: To minimize the risk of ototoxicity, auditory function monitoring should be considered in patients receiving concomitant ototoxic drugs.
- Laboratory Monitoring: Monitor renal function for nephrotoxicity. Periodic monitoring of white blood cell count should be done to screen for neutropenia in patients on prolonged therapy with vancomycin or those who are receiving concomitant drugs that may cause neutropenia. Monitor for infusion-related events, including hypotension and red man syndrome.
- Vancomycin Concentration:
- Trough: Troughs should be obtained just prior to the next dose under steady state conditions (approximately just before the fourth dose) and then repeated as clinically necessary. Trough concentrations (not peak) are the most accurate measure to monitor for efficacy . Due to the variability in pharmacokinetic parameters, peak and trough concentrations have been recommended to provide more individualized dosing in neonates . If peak concentrations are measured, draw 60 minutes after end of infusion.
- Target Concentration: Neither vancomycin troughs nor AUC have been correlated with clinical outcomes in neonates. Multiple pharmacokinetic/pharmacodynamic studies in neonates evaluated AUC and MIC and determined vancomycin troughs of around 10 mg/L (range, 7 to 15 mg/L) for MICs of 1 mg/L or less may be adequate for the treatment of the most common neonatal gram-positive infections, which is predominately coagulase-negative staphylococcus. Although, higher troughs have been recommended in adults and children (older than neonates). For endocarditis in children (older than neonates), in the presence of MRSA with MIC of greater than 1 mg/L or when there is a lack of microbiological response, troughs of 15 to 20 mg/L may be required . In adults, many experts recommend a trough of 15 to 20 mg/L when treating MRSA bacteremia, infective endocarditis, osteomyelitis, meningitis, pneumonia, complicated skin and soft-tissue infections, or bone/joint infections. The recommended trough concentration range for adults with less severe infections is 10 to 15 mg/L.
- Shunt infections: For shunt infections, consider monitoring CSF vancomycin levels during therapy to assess drug concentrations (goal: trough, 5 to 10 mg/L) and potential drug accumulation . Methicillin-resistant Staphylococcus aureus isolates with a vancomycin MIC greater than 2 mg/L (eg, vancomycin-intermediate or vancomycin-resistant S. aureus [VISA or VRSA]) require alternative therapy
- Nephrotoxicity: The use of higher doses to achieve vancomycin trough concentrations of 15 to 20 mg/L and the association with nephrotoxicity is conflicting. The incidence of acute kidney injury (AKI) increased with higher vancomycin trough concentrations in 110 neonates during a prospective study (p=0.04); 1.38% for less than 10 mg/L, 0% for 10 to 15 mg/L, and 18.18% for greater than 15 mg/L. The odds of AKI increased by 16% for each 5 mg/kg/day increase in vancomycin dose in one pediatric retrospective study (n=175). But in another retrospective pediatric study (n=113), vancomycin trough concentrations of 15 mg/L or more were not associated with an increased rate of nephrotoxicity . Increased duration of vancomycin therapy was associated with increased risk of nephrotoxicity in the 2 retrospective studies. The definitions for nephrotoxicity used in these studies may be one reason for the differences in findings
