Infant Data
Results
INDICATIONS
- Isoimmune hemolytic disease : Immune globulin is recommended for isoimmune hemolytic disease in infants refractory to phototherapy (continued elevations in bilirubin or total bilirubin within 2 to 3 mg/dL of the exchange level). The efficacy of immune globulin was inconclusive for the treatment or prophylaxis of Rh or ABO hemolytic disease of the newborn in a meta-analysis (n=12 studies; 813 preterm and term infants).
- Measles exposure: Immune globulin, either IV or IM, is recommended in individuals with no evidence of immunity to measles. The IV immune globulin preparation is recommended in the following individuals.
Severely immunocompromised hosts regardless of immunologic or vaccination status, including the following:
- Patients with severe primary immunodeficiency
- Patients who have received a bone marrow transplant until at least 12 months after finishing all immunosuppressive treatment, or longer in patients who have developed graft-versus-host disease
- Patients on treatment for acute lymphoblastic leukemia within and until at least 6 months after completion of immunosuppressive chemotherapy
- Patients with HIV infection or AIDS who have severe immunosuppression defined as CD4+ T-lymphocyte percentage of less than 15% (all ages) and those who have not received the measles, mumps, rubella vaccine since receiving effective antiretroviral therapy.
- Neonatal alloimmune thrombocytopenia: The recommendation to use immune globulin for neonatal alloimmune thrombocytopenia is conflicting.
- Sepsis: Neither mortality nor major disability at the age of 2 years was reduced with adjunctive IV immune globulin administered to neonates with proven or suspected serious infection and weighing less than 1500 g in a double-blind, randomized, controlled trial (n=3493). The dose of IV immune globulin was 500 mg/kg followed by a second dose 48 hours later.
- neonatal alloimmune neutropenia, parvovirus B19 infection, and Kawasaki disease: There is not enough evidence to use immune globulin for this conditions in neonates.
FDA APPROVED INDICATION
- Intramuscular:
- Safety and effectiveness in pediatric patients have not been established.
- Intravenous(vary by specific product):
- For the treatment of primary immunodeficiency diseases (PIDD). Gammagard® Liquid, Gammagard® S/D, and Gammaplex® are approved in children 2 years of age and older for PIDD. Privigen® is approved in children 3 years of age or older for PIDD. Bivigam® is approved in children 6 years or older for PID.
- For the treatment of idiopathic thrombocytopenic purpura (ITP). Privigen® is approved for ITP in patients 15 years of age or older.
- Gammaked™ is approved for the treatment of inflammatory demyelinating polyneuropathy (CIDP).
- Gammagard® S/D is approved for the treatment of Kawasaki syndrome.
- Subqutaneous:
- For the treatment of primary immunodeficiency disease in pediatric patients 2 years or older. This includes, but is not limited to, common variable immunodeficiency, Xlinked agammaglobulinemia, congenital agammaglobulinemia, Wiskott-Aldrich syndrome, and severe combined immunodeficiencies.
CONTRAINDICATIONS
Anaphylaxis or severe systemic reaction to human immunoglobulins or to any component of the product, including polysorbate 80 Hereditary intolerance to fructose, including infants and neonates for whom sucrose or fructose tolerance has not been established Hyperprolinemia (type I or II); Hizentra(R) and Privigen(R) contain the stabilizer L-proline. IgA deficiency with antibodies against IgA, and a history of hypersensitivity; IG productscontain trace amounts of IgA Severe thrombocytopenia or any coagulation disorder which would contraindicate IM injections
PRECAUTIONS
- Dosing:
- Expanded fluid volume may cause overload with high-dose regimens for chronic idiopathic thrombocytopenic purpura in patients at increased risk of acute kidney injury, hemolysis, thrombosis, or volume overload.
- Endocrine & Metabolic:
- Falsely elevated glucose measurements may occur during therapy in diabetic patients because of the maltose ingredient. This increases the risk of masked hypoglycemic episodes and over administration of insulin, potentially causing lifethreatening hypoglycemia .
- Hyperproteinemia, increased serum viscosity, and hyponatremia may occur. Distinguish hyponatremia from pseudohyponatremia (decreased calculated serum osmolality or elevated osmolar gap).
- Hyperproteinemia, increased serum viscosity, and hypernatremia or pseudohyponatremia may occur with Gammagard S/D due to amount of sodium in product.
- Hematologic:
- Hemolysis and delayed hemolytic anemia may occur. Severe hemolysisrelated renal dysfunction, renal failure, and disseminated intravascular coagulation have been reported. Increased risk with high doses (2 g/kg or greater), non-O blood group, and underlying inflammation.
- Immunologic:
- Severe hypersensitivity reactions have been reported. Increased risk in IgA deficiency with anti-IgA antibodies or corn allergy. Discontinue use if condition occurs.
- Infusion reactions (ie, fever, chills, nausea, and vomiting) may occur, especially with first dose or with treatment hiatus of more than 8 weeks. Adherence to dose and administration guidelines recommended.
- Infectious agent transmission may occur, including viruses and theoretical risk of Creutzfeldt-Jakob disease, as well as unknown or emerging viruses and other pathogens.
- A false positive skin test may occur when an intradermal injection of concentrated gamma globulin solution is administered; do not perform skin tests.
- Neurologic:
- Aseptic meningitis syndrome (AMS) may occur. Increased risk with high doses (1 to 2 g/kg or greater) or rapid infusion. Discontinuation may be necessary.Increased susceptibility to AMS may occur in patients with migraine history or in female patients.
- Renal:
- Acute renal dysfunction, renal failure, acute tubular necrosis, proximal tubular nephropathy, osmotic nephrosis, and death may occur with immune globulin products, especially those that contain sucrose. Dosage adjustment may be necessary, particularly in patients with increased risk (eg, developing renal dysfunction, preexisting renal insufficiency, iabetes mellitus, volume depletion, sepsis, paraproteinemia, or concomitant nephrotic drugs).
- Respiratory:
- Transfusion-related acute lung injury (noncardiogenic pulmonary edema) may occur, usually with presenting symptoms within 1 to 6 hours of treatment.
- Laboratory Interference:
- False-positive readings may occur in assays dependent on detection of beta-D-glucans for diagnosis of fungal infection
ADVERSE EFFECTS
Intravenous: Rare cases of hypoglycemia, transient tachycardia, and hypotension that resolved after stopping the infusion have been reported. The risk of necrotizing enterocolitis may be increased in term and late preterm infants treated for isoimmune hemolytic jaundice. Animal studies have demonstrated reticuloendothelial system blockade when higher doses (greater than 1 g/kg) have been used. All donor units are nonreactive to HBsAg and HIV. The manufacturing process of these products now includes a solvent/detergent treatment to inactivate hepatitis C and other membrane-enveloped viruses.
BLACK BOX WARNING
Renal Dysfunction and Failure: Renal dysfunction, acute renal failure, osmotic nephrosis, and death have been reported. Increased risk with concomitant use of nephrotoxic drugs, preexisting renal insufficiency, diabetes mellitus, volume depletion, sepsis, or paraproteinemia. Discontinuation may be necessary. Renal dysfunction and acute renal failure are more common with use of immune globulin IV products that contain sucrose; glucose-free products include Bivigam(TM), Flebogamma(R) 5% DIF, Flebogamma(R) 10% DIF, Gammaplex(R), Gammagard Liquid(R), Gammagard S/D, Gammaked(TM), Gamunex(R)-C, Octagam(R) 10%, Privigen(R), and Hizentra(R) Thrombosis:Thrombosis may occur with immune globulin products. Risk factors may include prolonged immobilization, hypercoagulable conditions, history of venous or arterial thrombosis, use of estrogens, indwelling central vascular catheters, hyperviscosity, and cardiovascular risk factors. Thrombosis may occur in the absence of known risk factors. For patients at risk of thrombosis, administer at the minimum dose and infusion rate practicable, ensure adequate hydration before administration, and monitor for thrombosis. Assess blood viscosity in patients at risk for hyperviscosity.
ADMINISTRATION
IM immune globulin and IV immune globulin are not interchangeable.
- Intramuscular: Do not administer by subQ or IV. Do not administer in the gluteal region. Administer in the anterolateral aspects of the upper thigh and deltoid muscle of the upper arm. Do not administer at the same time as measles vaccine.
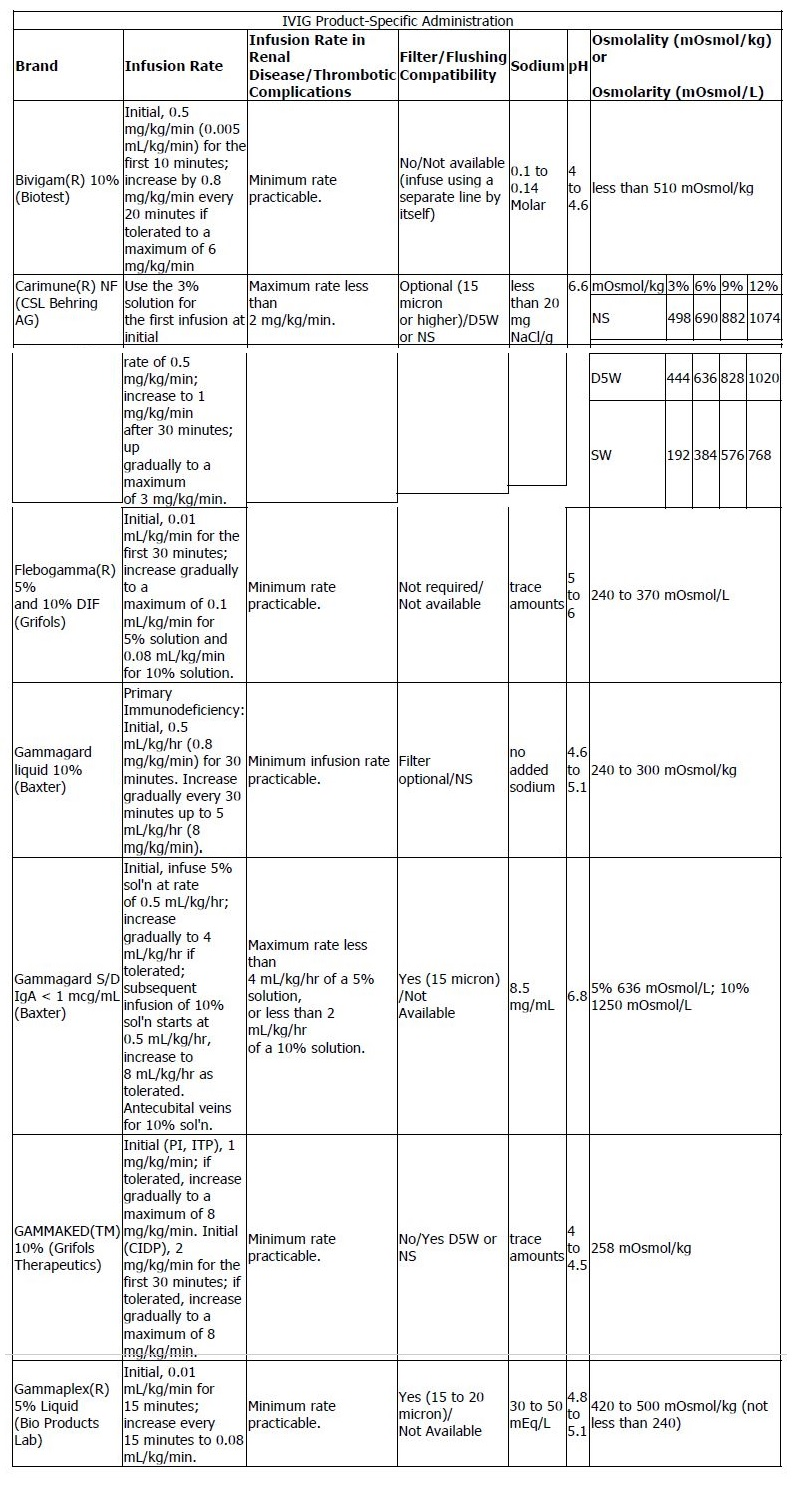
- Intravenous: Selection of product depends on osmolarity, pH, viscosity, volume administered, and sodium or sugar content. Rate of administration varies by product; refer to the IVIG Product-Specific Administration table below for specific information.

MONITORING
Intramuscular: Baseline assessment of blood viscosity should be considered in those at risk for hyperviscosity. Intravenous: Monitor carefully the rate of infusion for the first and possibly the second infusion due to adverse effects. Monitor vital signs closely during infusion. Periodic assessment of CBC, renal function, and urine output is recommended during therapy. Monitor for signs of hemolysis and thrombotic events, especially in patients with known risk factors. Baseline assessment of blood viscosity should be considered in those at risk for hyperviscosity.
