Infant Data
PMA= gestational age plus postnatal age
Results
INDICATIONS
Bacteriostatic antibiotic used for the treatment of bacteremia and pulmonary and deep tissue infections caused by anaerobic bacteria and some gram-positive cocci. Clindamycin should not be used in the treatment of meningitis due to inadequate perfusion into the CSF
- Anthrax:
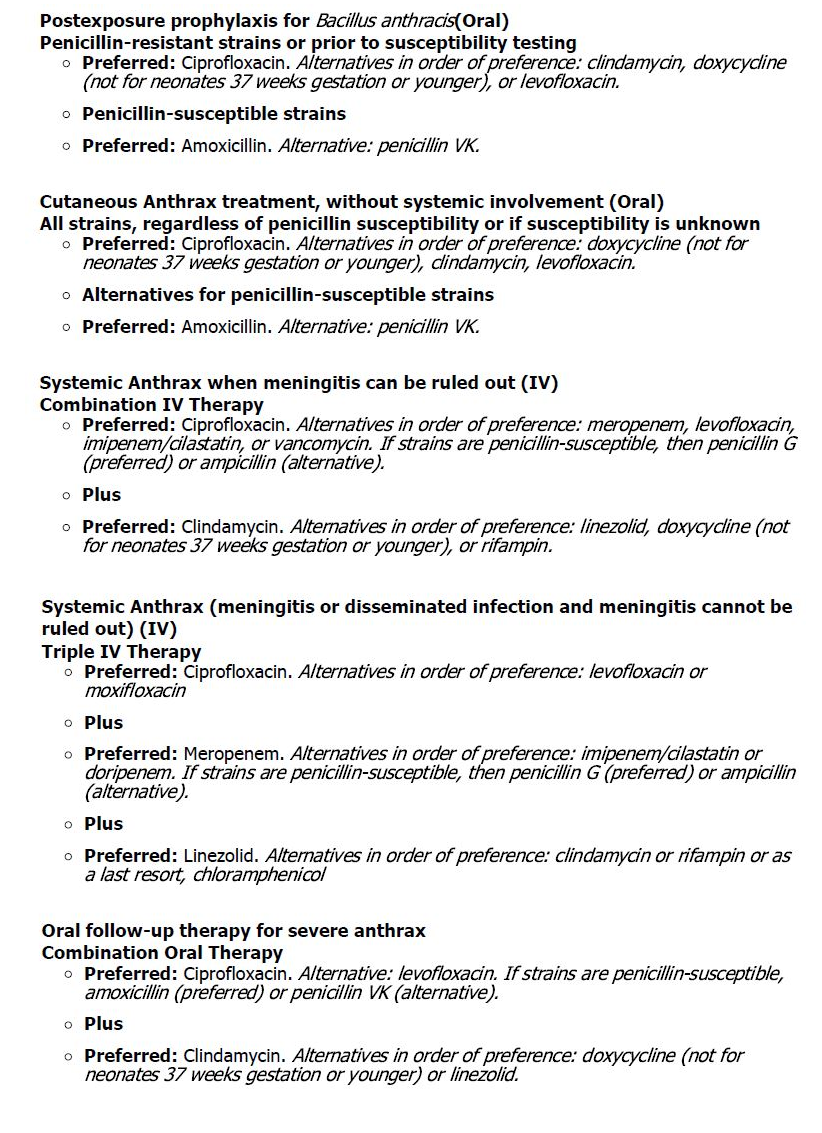
CONTRAINDICATIONS
Contraindicated in patients with a history of lincomycin allergies.
PRECAUTIONS
- Concomitant use with erythromycin is not recommended.
- Exercise caution in patients with a history of gastrointestinal disease, particularly colitis.
- Anaphylactic and severe hypersensitivity reactions (eg, toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms, and Steven-Johnson syndrome), sometimes fatal, have been reported. Discontinue use upon occurrence.
- Use cautiously in patients with a history of atopy.
- Superinfections may occur, especially yeast overgrowth.
- Not recommended for treatment of meningitis. Clindamycin does not adequately diffuse into CSF
ADVERSE EFFECTS
Hypersensitivity reactions, and jaundice and liver function test abnormalities have been reported in association with clindamycin therapy. Should not be used in combination with topical or oral erythromycin-containing products due to possible antagonism.
BLACK BOX WARNING
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including clindamycin, and may range in severity from mild to life-threatening. Because clindamycin therapy has been associated with severe colitis which may end fatally, it should be reserved for serious infections where less toxic antimicrobial agents are inappropriate. Diarrhea, colitis, and pseudomembranous colitis have been observed to begin up to several weeks following cessation of therapy with clindamycin. If pseudomembranous colitis is suspected or confirmed, consider discontinuation of clindamycin and initiate appropriate fluid and electrolyte management, protein supplementation, C. difficile antibiotic treatment, and surgical evaluation as clinically indicated.
ADMINISTRATION
Infuse IV over 10 to 60 minutes, not to exceed 30 mg/min, at a concentration not to exceed 18 mg/mL. The recommended standard concentration for neonates is 6 mg/mL.
MONITORING
Assess liver function. Monitor GI status closely. Therapeutic serum concentration ranges from 2 to 10 mcg/mL (bioassay yields variable results).
