CALCUTIONS AREA
CLICK ON CALCULATOR
Infant Data
Results
MEDICAL INFORMATIONS
INDICATIONS
- Disseminated gonococcal infections and gonococcal scalp abscesses: The recommended regimen is ceftriaxone or cefotaxime.
- Infective Endocarditis: The following recommendations are based on a consensus of experts . The full pediatric guidelines can be found here:
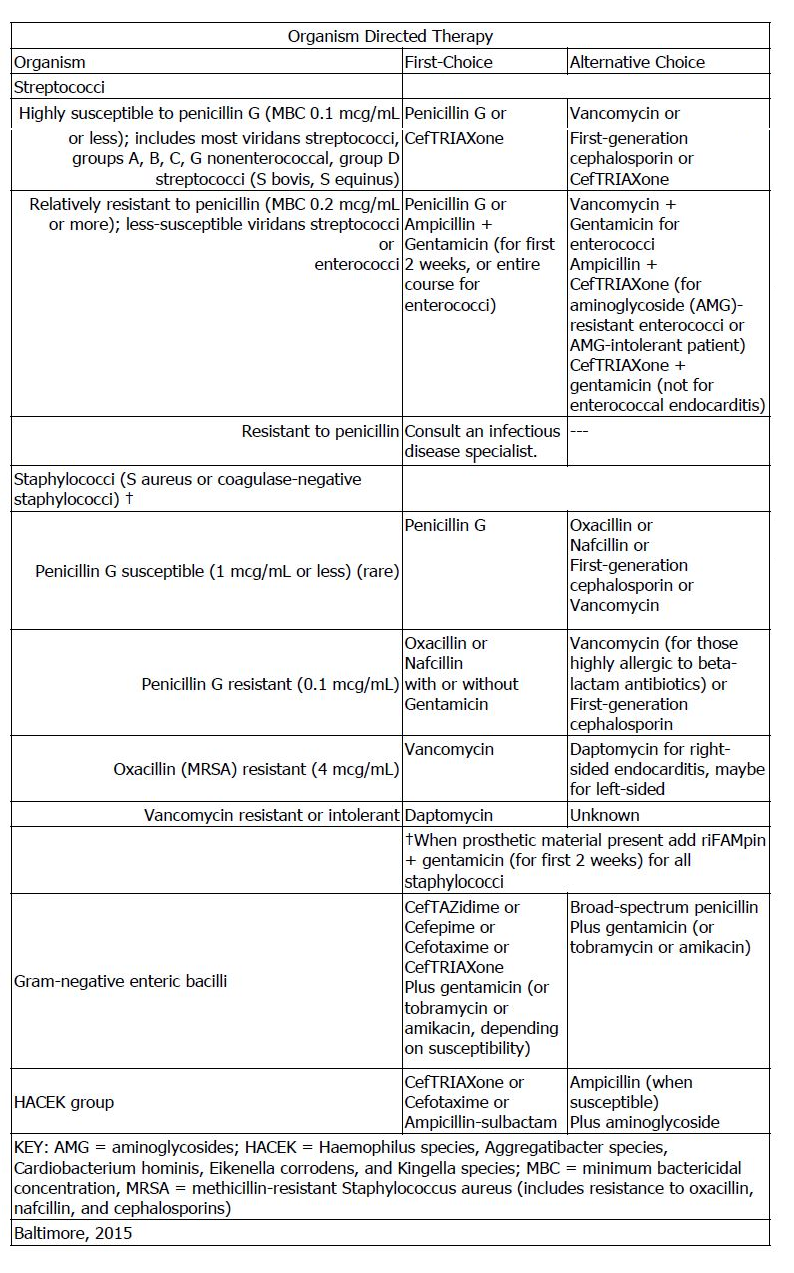
- Meningitis: Empiric agents for the treatment of meningitis in neonates are ampicillin, gentamicin, and cefotaxime. Reassess therapy based on culture and sensitivity results
- Sepsis: caused by susceptible gram-negative organisms (e.g. E coli, H influenzae,and Klebsiella). Optimal treatment for suspected, early-onset sepsis is broad-spectrum antimicrobial coverage using a combination of ampicillin and an aminoglycoside (usually gentamicin); once a pathogen is identified, therapy should be narrowed unless synergism is required
- Duration: Procalcitonin values in addition to perinatal risk factors, signs and symptoms, and laboratory values may aid in the determination to discontinue antibiotic therapy in neonates with suspected early-onset sepsis. The duration of antibiotic therapy was reduced by 9.9 hours with a procalcitonin-guided algorithm compared with standard care in a multicenter randomized control trial of 1710 neonates born after 34 weeks of gestational age with possible or unlikely sepsis. Re-infection and mortality was not different between the groups (risk difference 0.1% (95% CI, -5.2% to 5.3%))
FDA APPROVED INDICATION
- Lower respiratory tract infections (including pneumonia): caused by Streptococcus pneumoniae, S pyogenes and other streptococci (excluding enterococci), Staphylococcus aureus (penicillinase and non-penicillinase producing), Escherichia coli, Klebsiella species, Haemophilus. influenzae (including ampicillin resistant strains), H parainfluenzae, Proteus mirabilis, Serratia marcescens, Enterobacter species, indole positive Proteus and Pseudomonas species (including P aeruginosa).
- Genitourinary infections (urinary tract infections): caused by Enterococcus species, S epidermidis, S aureus (penicillinase and non-penicillinase producing), Citrobacter species, Enterobacter species, E coli, Klebsiella species, P mirabilis, P vulgaris, P stuartii, M. morganii, P rettgerr, S marcescens, and Pseudomonas species (including P. aeruginosa). Also uncomplicated gonorrhea (cervical/urethral and rectal) caused by N. gonorrhoeae, including penicillinase-producing strains.
- Bactremia/Sepsis: caused by E coli, Klebsiella species, and S marcescens, S aureus, and Streptococcus species (including S pneumoniae).
- Skin and skin structure infections: caused by S aureus (penicillinase and non-penicillinase producing), S epidermidis, S pyogenes and other streptococci, Enterococcus species, Acinetobacter species, E. coli, Citrobacter species (including C freundii), Enterobacter species, Klebsiella species, P. mirabilis, P vulgaris, M. morganii, P rettgerr, Pseudomonas species, S marcescens, Bacteroides species, and anaerobic cocci (including Peptostreptococcus species and Peptococcus species).
- Intra-Abdominal Infections: caused by Streptococcus species, E. coli, Klebsiella species, Bacteroides species, anaerobic cocci (including Peptostreptococcus species and Peptococcus species), P mirabilis, and Clostridium species. Cefotaxime plus metronidazole is considered an appropriate combination antibiotic regimen for pediatric patients with a complicated extra-biliary intra-abdominal infection.
- Bone and/or Jont Infections: caused by S aureus (penicillinase and non-penicillinase producing), Streptococcus species (including S pyogenes), Pseudomonas species (including P aeruginosa), and P mirabilis.
- Central nervous system infections (eg, meningitis and ventriculitis): caused by N meningitidis, H influenzae, S pneumoniae, K pneumoniae, and E coli.
PRECAUTIONS
- Extravasation:
- including extensive perivascular, may occur causing tissue damage requiring surgical intervention. Use cautiously in patients with a history of gastrointestinal disease, especially colitis.
- lostridium difficile-associated diarrhea:
- ranging from mild diarrhea to fatal colitis, has been reported. Discontinuation may be required.
- Hematologic:
- Leukopenia, neutropenia, or granulocytopenia and in rare cases bone marrow failure, pancytopenia, or agranulocytosis may occur.
- Immunologic:
- Increased risk of allergic reaction including serious reactions requiring medical intervention in patients with previous hypersensitivity to penicillins, other drugs, or other demonstrated allergy.
- Drug Resistancy:
- Drug-resistant bacteria may develop if used in the absence of bacterial infection.F
- Renal:
- Use caution in the presence of renal insufficiency. Dose reductions are recommended with CrCL less than 20 mL/min/1.73 m(2).
- Rapid bolus injection:
- Rapid bolus injection via a central venous catheter has resulted in life-threatening arrhythmias.
- Laboratory:
- A false-positive reaction for urine glucose may occur with copper reduction test. Enzymebased tests for glycosuria are recommende
ADVERSE EFFECTS
Side effects are rare but include rash, phlebitis, diarrhea, leukopenia, granulocytopenia, and eosinophilia. In a prospective cohort study (n=4579), third generation cephalosporins started by day 3 of life in extremely low birth weight infants (less than 1000 g) were associated with a significantly increased risk of candidiasis compared with other antibiotics.
ADMINISTRATION
May be given by IM injection, IV push (over 3 to 5 minutes), or intermittent IV infusion. For IV push, a concentration of 50 to 100 mg/mL may be used. For intermittent IV infusion, dilute to a concentration of 10 to 40 mg/mL and infuse over 10 to 30 minutes.
MONITORING
Measuring serum concentration is not usually necessary. Periodic CBC.
